Spartina®: Proven Weight Reduction for Adults with Obesity or Overweight


The accompanying image is for illustrative purposes only and does not depict study participants.
Clinically Proven Body Weight Reduction with tirzepatide
In the 72‑week SURMOUNT‑1 trial, tirzepatide 15 mg delivered weight reductions approximately 7 times greater than placebo.*
Adults lost an average of
20.9%
of their body weight with Spartina 15 mg vs 3.1% with placebo.*
Overall Percentage Change in Body Weight from Baseline at 72 Weeks with tirzepatide
-15.0%
(-15.4 kg)
Tirzepatide 5 mg
(n=630)
From a mean baseline of 102.9 kg
-19.5%
(-20.1 kg)
Tirzepatide 10 mg
(n=636)
From a mean baseline of 105.8 kg
-20.9%
(-21.8 kg)
Tirzepatide 15 mg
(n=630)
From a mean baseline of 105.6 kg
-3.1%
(-2.9 kg)
Placebo
(n=643)
From a mean baseline of 104.8 kg
Graphic showing the overall percentage change in body weight from baseline in the SURMOUNT-1 trial (tirzepatide 5 mg, 10 mg, and 15 mg vs placebo at 72 weeks).
It shows the average percentage change in body weight from baseline after 72 weeks for four groups:
- tirzepatide 5 mg: –15.0% weight reduction
- tirzepatide 10 mg: –19.5% weight reduction
- tirzepatide 15 mg: –20.9% weight reduction
- Placebo: –3.1% weight reduction
Supporting context:
- The number of participants in each group was around 630–643.
- Mean baseline body weight across all participants was approximately 105 kg.
- It emphasizes dose-dependent weight loss: higher tirzepatide doses showed greater percentage reductions.
Comprehensive Care Approach for Overweight and Obesity Management
Weight management should be personalized rather than approached with a “one-size-fits-all” strategy, with treatment intensity aligned to the severity of obesity-related complications.
Evidence-based obesity care begins with tailored lifestyle interventions—diet, physical activity, and behavioral therapy—with pharmacologic treatments introduced as needed.
مکانیسم اثر
Spartina (tirzepatide) is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. This dual action is specifically designed to address both glycemic control and weight management.
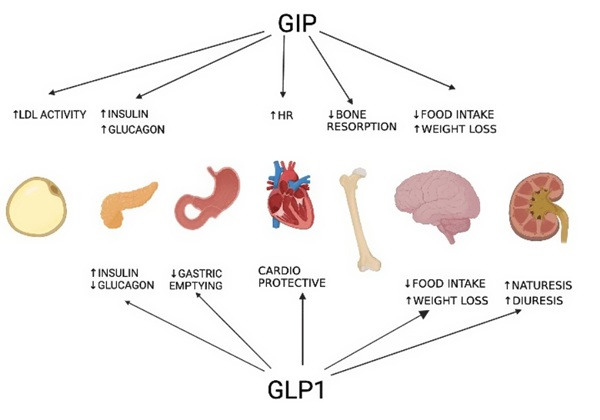
In the context of weight management, Spartina contributes through:
- Appetite Regulation: Spartina activates GLP-1 and GIP receptors involved in satiety signaling, helping reduce hunger and calorie intake.
- Delayed Gastric Emptying: By slowing gastric emptying, Spartina prolongs feelings of fullness, which supports reduced meal sieze and frequency.
- Energy Balance Modulation: Spartina promotes weight loss by influencing central nervous system pathways linked to food intake regulation and energy expenditure.
- Fat Mass Reduction: Clinical data show tirzepatide reduces body weight primarily through fat mass loss while preserving lean body mass.
Proven Efficacy in Weight Management
Tirzepatide is a dual GIP and GLP‑1 receptor agonist approved for chronic weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) who have at least one weight‑related comorbidity (e.g., hypertension, dyslipidaemia or type 2 diabetes), in combination with a reduced‑calorie diet and increased physical activity. Tirzepatide produced statistically significant and clinically meaningful weight reduction in 5 SURMOUNT trials
Weight Reduction in Adults Without Type 2 Diabetes
In SURMOUNT‑1, once‑weekly tirzepatide produced significant and sustained weight loss compared to placebo after 72 weeks.
Proportion of patients achieving ≥5% weight loss at 72 weeks:
- Tirzepatide 15 mg: 90.9%
- Tirzepatide 10 mg: 88.9%
- Tirzepatide 5 mg: 85.1%
- Placebo: 34.5%
Weight Reduction in Adults With Type 2 Diabetes
In SURMOUNT‑2, 72 weeks treatment with tirzepatide demonstrated clinically meaningful body weight reductions in type 2 diabetes patients.
Proportion of patients achieving ≥5% weight loss at 72 weeks:
- Tirzepatide 15 mg: 86.4%
- Tirzepatide 10 mg: 81.6%
- Placebo: 30.6%
In SURMOUNT‑1, 50.1% of participants receiving tirzepatide 10 mg and 56.7% receiving 15 mg lost ≥20% of their baseline body weight at week 72.
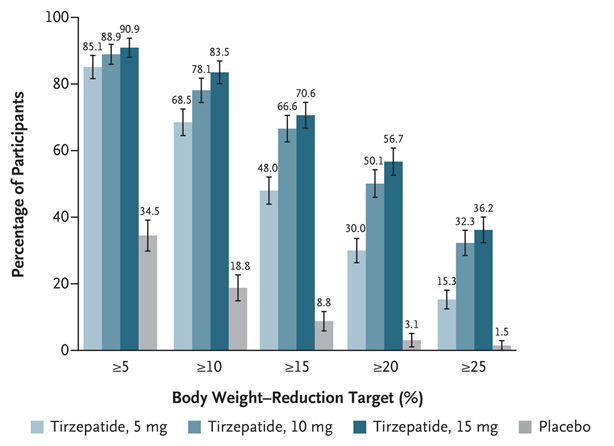
Tirzepatide demonstrated significantly superior efficacy in weight reduction compared to placebo across all measured endpoints.
A clear dose-response relationship was observed, with higher doses of tirzepatide leading to a greater percentage of participants achieving more substantial weight loss.
The data clearly indicates that tirzepatide is highly effective for weight reduction, with its efficacy increasing with higher doses. All tirzepatide dosage groups significantly outperformed placebo across all weight reduction thresholds, demonstrating its potential as a treatment for weight management. Notably, a substantial proportion of participants on higher tirzepatide doses achieved clinically significant weight loss of 20% or more.
Improvements in cardiometabolic parameters with tirzepatide
TRIGLYCERIDES
tirzepatide baseline=127.5 mg/dL
Placebo baseline=130.8 mg/dL
-5.6% with placebo
LDL CHOLESTEROL
tirzepatide baseline=110.1 mg/dL
Placebo baseline=109.4 mg/dL
-1.7% with placebo
HDL CHOLESTEROL
tirzepatide baseline=47.6 mg/dL
Placebo baseline=46.6 mg/dL
-0.7% with placebo
DIASTOLIC BLOOD PRESSURE
tirzepatide baseline=79.5 mm Hg
Placebo baseline=79.6 mm Hg
-0.8 mm Hg with placebo
SYSTOLIC BLOOD PRESSURE
tirzepatide baseline=123.5 mm Hg
Placebo baseline=122.9 mm Hg
-1.0 mm Hg with placebo
Head-to-Head Comparison: Tirzepatide vs. Semaglutide
In the SURMOUNT‑5 trial comparing tirzepatide with semaglutide, tirzepatide demonstrated significantly greater weight loss over 72 weeks (mean change −20.2% vs −13.7%).
These data suggest tirzepatide can be a more effective weight‑management therapy in adults with obesity.
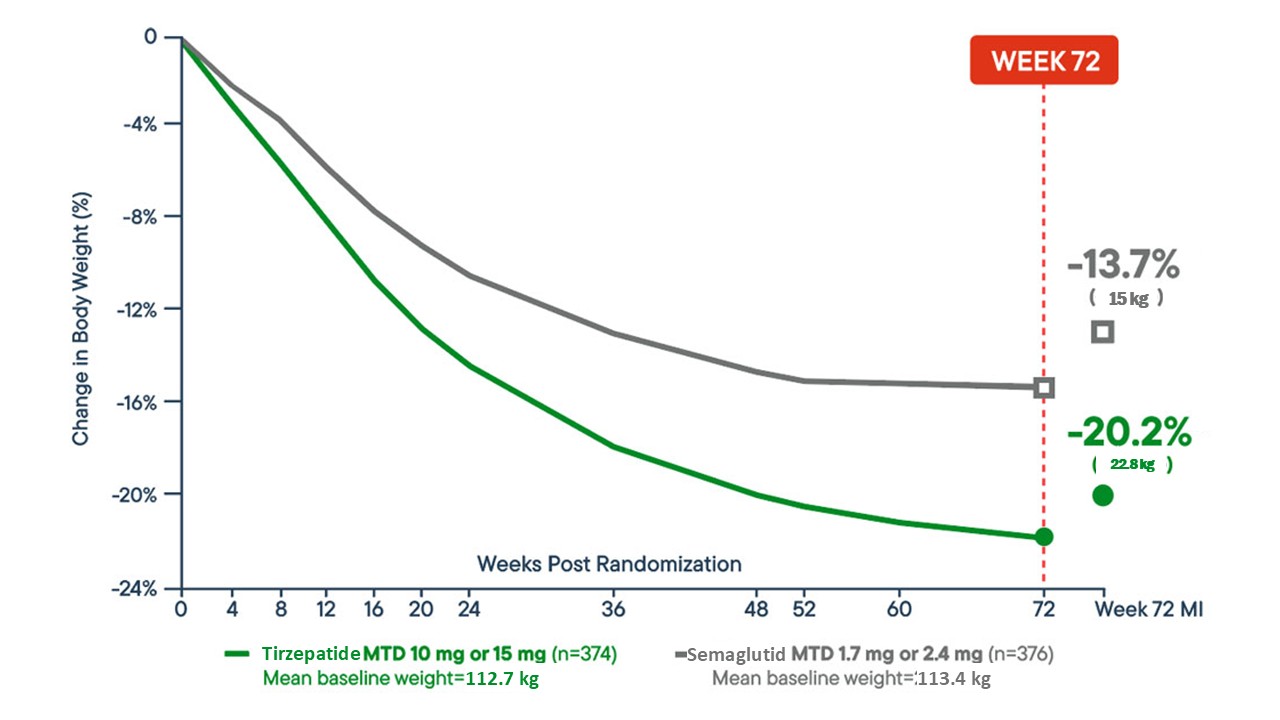
Percentage Change in Body Weight: Spartina (Tirzepatide) vs. Semaglutide at 72 Weeks
A line graph from the SURMOUNT-5 trial illustrates the percentage change in body weight from baseline to week 72:
- Spartina maximum tolerated dose (MTD: 10 mg or 15 mg)
- n = 374 adults
- Mean percentage change: −20.2%
- Mean weight reduction: −50.3 lbs
- Semaglutide maximum tolerated dose (MTD: 1.7 mg or 2.4 mg)
- n = 376 adults
- Mean percentage change: −13.7%
- Mean weight reduction: −33.1 lbs
This head-to-head comparison highlights a greater overall body weight reduction with tirzepatide versus semaglutide over 72 weeks.
دوز مصرف و نحوه تجویز
Starting Dose: 2.5 mg subcutaneously once weekly for 4 weeks. Escalation: Increase in 2.5 mg increments every 4 weeks based on tolerance and clinical response. Note: The 2.5 mg dose is for initiation only and not a maintenance dose.
Choose maintenance dose according to patient's clinical response and tolerability. Maximum dose: 15 mg weekly.
Must be injected subcutaneously once weekly in the abdomen, thigh, or upper arm.
Select Important Safety Information
Spartina (tirzepatide) is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC), as well as in those with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). It is also contraindicated in patients with known serious hypersensitivity to tirzepatide or any of its excipients. Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with tirzepatide use. Healthcare professionals should evaluate patient history thoroughly before prescribing Spartina and provide appropriate counseling regarding potential symptoms of hypersensitivity reactions.
Patients should be counseled about the potential risk for medullary thyroid carcinoma (MTC) when using Spartina (tirzepatide) and should be informed about symptoms that may indicate thyroid tumors, such as a mass in the neck, difficulty swallowing, shortness of breath, or persistent hoarseness. Routine monitoring of serum calcitonin levels or thyroid ultrasound is not routinely recommended, as these methods have uncertain value for early MTC detection. Such monitoring may lead to unnecessary procedures due to low test specificity and the high background incidence of thyroid disease. Significantly elevated serum calcitonin values, typically above 50 ng/L, may indicate MTC and require further evaluation. Patients presenting with thyroid nodules upon physical examination or imaging should also undergo appropriate diagnostic assessment.
The use of Spartina (tirzepatide) has been associated with gastrointestinal adverse reactions, including events that may be severe. In a pooled analysis from two clinical trials (SURMOUNT-1 and SURMOUNT-2), severe gastrointestinal adverse reactions were reported more frequently in patients receiving Spartina compared to placebo. Specifically, severe gastrointestinal adverse reactions occurred in 1.7% of patients receiving 5 mg, 2.5% of patients receiving 10 mg, and 3.1% of patients receiving 15 mg, versus 1.0% in patients receiving placebo. Comparable rates of severe gastrointestinal adverse reactions were observed in clinical trials evaluating Spartina for weight reduction and for obstructive sleep apnea. Spartina has not been studied in patients with severe gastrointestinal diseases, including severe gastroparesis, and is therefore not recommended for use in these patients.
The use of Spartina (tirzepatide) has been associated with acute kidney injury, which may occur as a result of dehydration linked to gastrointestinal adverse reactions such as nausea, vomiting, and diarrhea. Postmarketing reports from patients treated with GLP-1 receptor agonists have included cases of acute kidney injury and worsening of chronic renal failure, some of which required hemodialysis. These events have been documented even in patients without previously known renal disease. In most reported cases, acute kidney injury occurred in the context of gastrointestinal symptoms leading to volume depletion. Renal function should be monitored in patients receiving Spartina, particularly if they report adverse reactions that could increase the risk of dehydration.
Ask ChatGPT
Treatment with Spartina (tirzepatide) is associated with an increased occurrence of acute gallbladder disease. In pooled data from two clinical trials (SURMOUNT-1 and SURMOUNT-2), cholelithiasis was reported in 1.1% of patients treated with Spartina compared to 1.0% of those receiving placebo. Cholecystitis occurred in 0.7% of Spartina-treated patients versus 0.2% of placebo-treated patients, and cholecystectomy was reported in 0.2% of Spartina-treated patients, with no cases reported in the placebo group. Acute gallbladder events were observed in association with weight reduction, and similar rates of cholelithiasis were documented in clinical trials evaluating Spartina for both weight reduction and obstructive sleep apnea. If cholecystitis is suspected during treatment, gallbladder diagnostic studies and appropriate clinical follow-up are recommended.
Use of Spartina (tirzepatide) has been associated with gastrointestinal adverse reactions, including events that may be severe. In a pooled analysis of two clinical trials (SURMOUNT-1 and SURMOUNT-2), severe gastrointestinal adverse reactions occurred more frequently in patients treated with Spartina compared to those receiving placebo. Specifically, severe gastrointestinal adverse reactions were reported in 1.7% of patients receiving 5 mg, 2.5% of patients receiving 10 mg, and 3.1% of patients receiving 15 mg of Spartina, compared to 1.0% of patients in the placebo group. Comparable rates of severe gastrointestinal adverse reactions were observed in clinical trials evaluating Spartina for weight reduction and for obstructive sleep apnea. Spartina has not been studied in patients with severe gastrointestinal diseases, including severe gastroparesis, and its use is not recommended in these individuals.
منابع
– Tirzepatide – Prescribing information
– Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216. doi:10.1056/NEJMoa2206038
– Garvey WT, Fras JP, Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT 2) a double blind, randomised, multicentre, placebo controlled, phase 3 trial. Lancet. 2023402(10402):613-626, doi:10.1016/50140-6736(23)01200-X
– Aronne LJ, Bade Horn D, le Roux CW, et al. Tirzepatide as compared with semaglutide for the treatment of obesity. N Engl J Med. 2025;Epub1-58. doi:10.1056/NEJMoa2416394
