Spartina®: A Novel Pharmacological Approach for Moderate-to-Severe Obstructive Sleep Apnea (OSA) in Adults with Obesity
Clinically Proven Efficacy of Tirzepatide in Reducing OSA Severity
Adults with moderate-to-severe OSA treated with tirzepatide achieved statistically significant reductions in Apnea-Hypopnea Index (AHI) compared to placebo*

The accompanying image is decorative and does not depict clinical evidence.
PRIMARY ENDPOINT: CHANGE IN AHI FROM BASELINE TO WEEK 52 (EVENTS/h)
SURMOUNT-OSA was a randomized, placebo-controlled trial evaluating tirzepatide in adults with obesity and moderate-to-severe obstructive sleep apnea.
The results demonstrated a significant mean reduction in the Apnea-Hypopnea Index (AHI) during 52 weeks of trial.
Study 1 - Not on PAP therapy
-50.7% Reduction in AHI from baseline in people taking tirzepatide MTD
vs
-3.0% with Placebo
In Study 1, tirzepatide was evaluated in adults with moderate-to-severe OSA and obesity (n=234) who were unable or unwilling to use positive airway pressure (PAP) therapy. All participants had discontinued PAP therapy for a minimum of 4 weeks prior to screening. Tirzepatide significantly reduced AHI in patients unable to use PAP, suggesting a pharmacologic option for those who cannot tolerate or access PAP therapy.
Study 2 - on PAP therapy
-58.7% Reduction in AHI from baseline in people taking tirzepatide MTD
vs
-2.5% with Placebo
In Study 2, tirzepatide was evaluated in adults with moderate-to-severe OSA and obesity (n=235) who had been on stable positive airway pressure (PAP) therapy for at least 3 consecutive months prior to screening, with the intention to maintain PAP therapy throughout the study. For efficacy assessments, participants suspended PAP use for 7 days prior to each scheduled polysomnography (PSG) to accurately measure the pharmacologic effect of Tirzepatide on apnea-hypopnea index (AHI) independent of PAP therapy.
In two pivotal studies evaluating tirzepatide in adults with moderate-to-severe obstructive sleep apnea (OSA) and obesity:
- In Study 1 (participants not using positive airway pressure [PAP] therapy), adults treated with tirzepatide at the maximum tolerated dose (MTD, n=114) achieved a mean absolute reduction of 25.3 apnea-hypopnea index (AHI) events per hour at week 52, compared to 5.3 events per hour with placebo (n=120). This corresponds to a 50.7% reduction in AHI from a baseline of 52.9 events per hour with tirzepatide versus a 3.0% reduction from a baseline of 50.1 events per hour with placebo.
- In Study 2 (participants on PAP therapy), adults receiving tirzepatide MTD (n=119) achieved a mean absolute reduction of 29.3 AHI events per hour at week 52, compared to 5.5 events per hour with placebo (n=114). This reflects a 58.7% reduction in AHI from a baseline of 46.1 events per hour with tirzepatide versus a 2.5% reduction from a baseline of 53.1 events per hour with placebo.
Up to half of adults taking tirzepatide achieved the criteria for OSA disease resolution
Disease resolution is defined as AHI <5 or AHI 5-14 without excessive sleepiness measured by ESS ≤101
% OF ADULTS WITH AHI <5 OR AHI 5-14 AND ESS ≤10 AT WEEK 52
Study 1 - Not on PAP Therapy
42.2%
tirzepatide MTD (n=114)
15.9%
Placebo (n=120)
Participants (n=234) who were unable or unwilling to use PAP therapy. Participants must not have used PAP for at least 4 weeks at the time of screening.
Study 2 - On PAP Therapy
50.2%
tirzepatide MTD (n=119)
14.3%
Placebo (n=114)
Participants (n=235) were on PAP therapy for at least 3 consecutive months at the time of screening and planned to continue PAP therapy during the study. Participants in Study 2 suspended PAP use for 7 days before the scheduled PSGs.
In two 52-week clinical studies of tirzepatide, adults with moderate-to-severe obstructive sleep apnea (OSA) and a BMI ≥30 kg/m² were randomized to receive tirzepatide at the maximum tolerated dose (10 mg or 15 mg) or placebo, both in combination with a reduced-calorie diet and increased physical activity.
Adults taking tirzepatide experienced significant reductions in hypoxic burden
CHANGE IN SLEEP APNEA-SPECIFIC HYPOXIC BURDEN FROM BASELINE TO WEEK 52
Study 1 - Not on PAP Therapy
-95.2%
min/h
tirzepatide MTD
(n=114)
From a mean baseline of 153.6% min/hr
-25.1%
min/h
Placebo
(n=120)
From a mean baseline of 137.8% min/hr
Participants (n=234) who were unable or unwilling to use PAP therapy. Participants must not have used PAP for at least 4 weeks at the time of screening.
Study 2 - On PAP Therapy
-103.0%
min/h
tirzepatide MTD
(n=119)
From a mean baseline of 132.2% min/hr
-41.7%
min/h
Placebo
(n=114)
From a mean baseline of 142.1% min/hr
Participants (n=235) were on PAP therapy for at least 3 consecutive months at the time of screening and planned to continue PAP therapy during the study. Participants in Study 2 suspended PAP use for 7 days before the scheduled PSGs.
In two 52-week clinical studies of tirzepatide in adults with moderate-to-severe obstructive sleep apnea (OSA) and obesity:
In Study 1 (participants not using PAP therapy), adults treated with tirzepatide at the maximum tolerated dose (MTD, n=114) experienced a 95.2% min/hour reduction in sleep apnea-specific hypoxic burden from a baseline of 153.6% min/hour, compared to a 25.1% min/hour reduction with placebo (n=120) from a baseline of 137.8% min/hour.
In Study 2 (participants on stable PAP therapy), adults receiving tirzepatide MTD (n=119) experienced a 103.0% min/hour reduction in hypoxic burden from a baseline of 132.2% min/hour, compared to a 41.7% min/hour reduction with placebo (n=114) from a baseline of 142.1% min/hour.
These findings demonstrate tirzepatide’s clinically meaningful impact on reducing sleep apnea-specific hypoxic burden, in addition to significant reductions in AHI, reinforcing its role as the first and only approved pharmacological therapy for moderate-to-severe OSA in adults with obesity.
مکانیسم اثر
Spartina® (tirzepatide) is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. Tirzepatide primarily improves OSA by reducing excess body weight, a key reversible risk factor
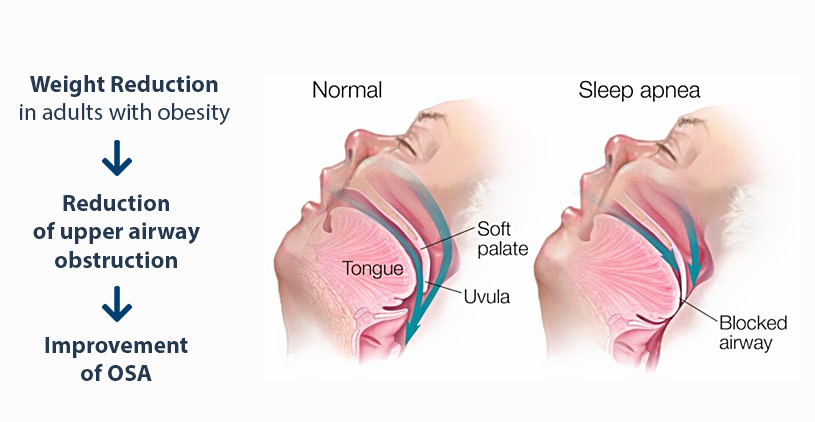
In the context of OSA, Spartina contributes through:
- Weight Reduction: Tirzepatide promotes significant weight loss through reduced appetite, delayed gastric emptying, and improved energy metabolism. Decreasing body weight reduces upper airway collapsibility, which is a core factor in OSA severity.
- Reduction of Upper Airway Obstruction: By lowering central and peripheral fat deposits, including visceral and upper airway adipose tissue, tirzepatide reduces pharyngeal fat volume, mitigating upper airway narrowing and collapsibility during sleep.
- Improved Respiratory Stability: Weight loss mediated by tirzepatide may reduce loop gain and enhance ventilatory control stability, contributing to fewer apneic events.
- Metabolic Modulation: Tirzepatide improves cardiometabolic risk factors (blood pressure, lipid profile and waist circumference), which may indirectly reduce cardiovascular comorbidities in OSA
دوز مصرف و نحوه تجویز
Starting Dose: 2.5 mg subcutaneously once weekly for 4 weeks.
Escalation: Increase in 2.5 mg increments every 4 weeks based on tolerance and clinical response.
Note: The 2.5 mg dose is for initiation only and not a maintenance dose.
Recommended Maintenance Dose: 10 mg or 15 mg subcutaneously once weekly.
Maximum Dose: 15 mg once weekly.
Must be injected subcutaneously once weekly in the abdomen, thigh, or upper arm.
Select Important Safety Information
Spartina® (tirzepatide) is contraindicated in individuals with a personal or family history of medullary thyroid carcinoma (MTC) or in those diagnosed with multiple endocrine neoplasia syndrome type 2 (MEN 2). It is also contraindicated in patients with known serious hypersensitivity to tirzepatide or any of its excipients. Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported in association with tirzepatide use.
Patients receiving Spartina® (tirzepatide) should be counseled regarding the potential risk of developing medullary thyroid carcinoma (MTC). Healthcare professionals should inform patients about the symptoms suggestive of thyroid tumors, including the presence of a neck mass, dysphagia, dyspnea, or persistent hoarseness. Routine monitoring of serum calcitonin concentrations or thyroid ultrasound for early detection of MTC in patients treated with Spartina® is not routinely recommended due to uncertain clinical value. This is attributed to the low specificity of calcitonin testing and a high background incidence of benign thyroid disease, which may lead to unnecessary diagnostic procedures. However, significantly elevated serum calcitonin levels, generally exceeding 50 ng/L, may indicate the presence of MTC and warrant further diagnostic evaluation. Similarly, patients presenting with thyroid nodules identified through physical examination or neck imaging should undergo appropriate clinical assessment.
The use of Spartina (tirzepatide) has been associated with gastrointestinal adverse reactions, including events that may be severe. In a pooled analysis from two clinical trials (SURMOUNT-1 and SURMOUNT-2), severe gastrointestinal adverse reactions were reported more frequently in patients receiving Spartina compared to placebo. Specifically, severe gastrointestinal adverse reactions occurred in 1.7% of patients receiving 5 mg, 2.5% of patients receiving 10 mg, and 3.1% of patients receiving 15 mg, versus 1.0% in patients receiving placebo. Comparable rates of severe gastrointestinal adverse reactions were observed in clinical trials evaluating Spartina for weight reduction and for obstructive sleep apnea. Spartina has not been studied in patients with severe gastrointestinal diseases, including severe gastroparesis, and is therefore not recommended for use in these patients.
The use of Spartina (tirzepatide) has been associated with acute kidney injury, which may occur as a result of dehydration linked to gastrointestinal adverse reactions such as nausea, vomiting, and diarrhea. Postmarketing reports from patients treated with GLP-1 receptor agonists have included cases of acute kidney injury and worsening of chronic renal failure, some of which required hemodialysis. These events have been documented even in patients without previously known renal disease. In most reported cases, acute kidney injury occurred in the context of gastrointestinal symptoms leading to volume depletion. Renal function should be monitored in patients receiving Spartina, particularly if they report adverse reactions that could increase the risk of dehydration.
Ask ChatGPT
Treatment with Spartina (tirzepatide) is associated with an increased occurrence of acute gallbladder disease. In pooled data from two clinical trials (SURMOUNT-1 and SURMOUNT-2), cholelithiasis was reported in 1.1% of patients treated with Spartina compared to 1.0% of those receiving placebo. Cholecystitis occurred in 0.7% of Spartina-treated patients versus 0.2% of placebo-treated patients, and cholecystectomy was reported in 0.2% of Spartina-treated patients, with no cases reported in the placebo group. Acute gallbladder events were observed in association with weight reduction, and similar rates of cholelithiasis were documented in clinical trials evaluating Spartina for both weight reduction and obstructive sleep apnea. If cholecystitis is suspected during treatment, gallbladder diagnostic studies and appropriate clinical follow-up are recommended.
Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported in patients treated with Spartina® (tirzepatide) during postmarketing surveillance. In pooled clinical data from the SURMOUNT-1 and SURMOUNT-2 trials, severe hypersensitivity reactions occurred in 0.1% of Spartina®-treated patients, with no such events reported in placebo-treated groups. Similar incidence rates were observed across clinical studies evaluating Spartina® for both weight management and obstructive sleep apnea (OSA). If signs or symptoms suggestive of a hypersensitivity reaction occur, patients should be advised to immediately seek medical attention and discontinue Spartina® therapy. Spartina® is contraindicated in individuals with a known history of serious hypersensitivity to tirzepatide or any of its excipients. Caution is advised when prescribing Spartina® to patients with a prior history of angioedema or anaphylaxis associated with other GLP-1 receptor agonists, as the predisposition for such reactions with Spartina® remains uncertain.
Spartina® (tirzepatide) lowers blood glucose levels and may cause hypoglycemia. In clinical trials involving patients with type 2 diabetes mellitus and a body mass index (BMI) ≥27 kg/m² (Study 2), hypoglycemia, defined as plasma glucose levels below 54 mg/dL, was reported in 4.2% of Spartina®-treated patients compared to 1.3% of patients receiving placebo. The incidence of hypoglycemia was notably higher in patients treated with Spartina® in combination with insulin secretagogues such as sulfonylureas, where the rate reached 10.3%, compared to 2.1% in Spartina®-treated patients not using sulfonylureas. An increased risk of hypoglycemia has also been observed in patients treated with tirzepatide in combination with insulin. Additionally, cases of hypoglycemia have been reported with Spartina® and other GLP-1 receptor agonists in adults without type 2 diabetes mellitus. Healthcare professionals should inform all patients about the risk of hypoglycemia and educate them on recognizing its signs and symptoms. In patients with diabetes mellitus, blood glucose levels should be monitored both prior to initiating Spartina® therapy and during treatment. To minimize the risk of hypoglycemia, dose adjustments of concomitantly administered insulin or insulin secretagogues, such as sulfonylureas, should be considered as appropriate.
منابع
References:
– Tirzepatide – Prescribing information
– Malhotra A, Grunstein RR, Fietze I, et al. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N Engl J Med. 2024;391(13):1193-1205. doi:10.1056/NEJMoa2404881
– Malhotra A, Grunstein RR, Fietze I, et al. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N Engl J Med. 2024; (suppl append). doi:10.1056/NEJMoa2404881
