Obesity is a chronic disease characterized by excessive adiposity and associated metabolic complications, including insulin resistance, type 2 diabetes, and cardiovascular risk. Effective weight management requires sustained reductions in energy intake and improvements in eating behavior, yet adherence to lifestyle interventions is often challenging. Pharmacological therapies that target both metabolic and behavioral drivers of obesity can offer substantial clinical benefits.
Tirzepatide, a dual glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) receptor agonist—or “twincretin”—has demonstrated superior efficacy in weight reduction and metabolic improvement compared with GLP-1 receptor agonists alone. Its weight-lowering effects are mediated not only through enhanced insulin sensitivity and metabolic regulation but also by appetite suppression and reductions in food intake. To better understand its impact on eating behavior, a phase 1 trial evaluated tirzepatide’s effects on food cravings and preferences in adults with obesity undergoing dietary restriction.
Evidence from a Phase 1 Randomized Controlled Trial
An 18-week, double-blind, placebo-controlled phase 1 study enrolled 55 adults with obesity (BMI 30–45 kg/m²) and at least one metabolic impairment. Participants received once-weekly tirzepatide 15 mg (n = 27) or placebo (n = 28) alongside structured nutritional counseling. Food preferences and cravings were assessed using the Food Preference Questionnaire (FPQ) and the Food Craving Inventory (FCI) at baseline, week 8, and week 18.
Key Outcomes
Weight Loss and Energy Intake
• Tirzepatide-treated participants lost an average of 16.7 kg versus 8.3 kg with placebo at 18 weeks (p < 0.001).
• Energy intake during ad libitum lunch and dinner meals was reduced by an additional 856 kcal with tirzepatide, alongside significant reductions in overall appetite.
Food Preferences and Cravings
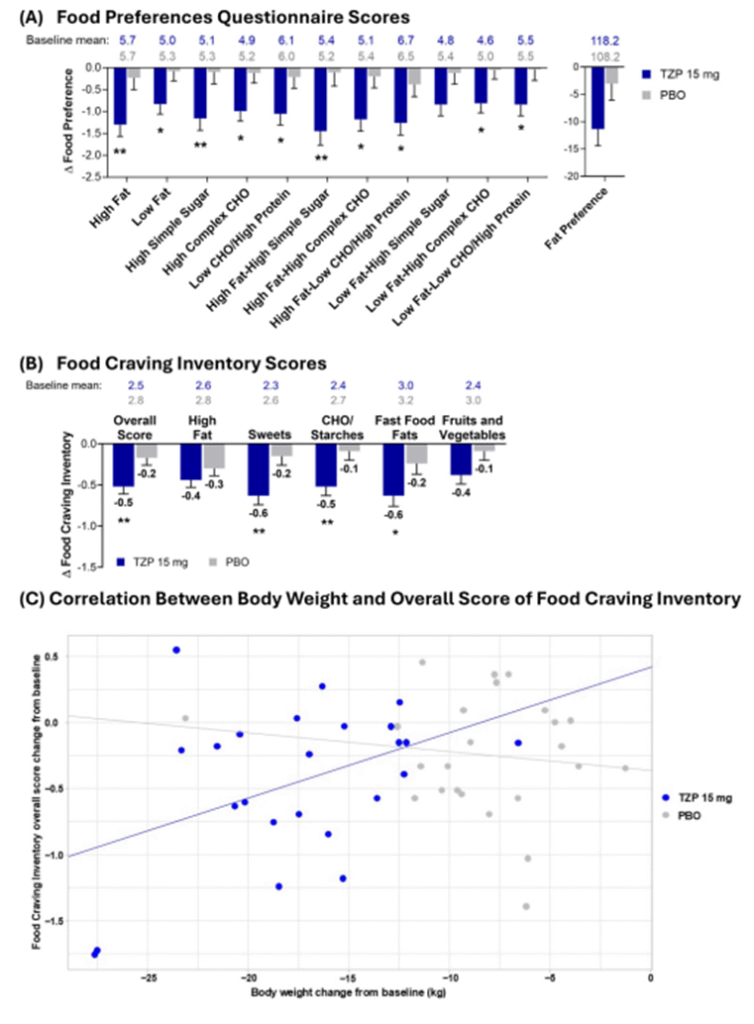
- Significant decreases in 10 of 12 FPQ scores were observed with tirzepatide compared with placebo at weeks 8 and 18 (p < 0.05). (Fig. A)
- Preferences for high-fat and high-simple-sugar foods were particularly reduced (p < 0.01).
- Tirzepatide lowered overall FCI scores and cravings for sweets, carbohydrates/starches, and fast-food fats (p < 0.05), while cravings for high-fat or fruits/vegetables items were unaffected. (Fig. B)
- Greater reductions in food cravings correlated with larger weight loss among tirzepatide-treated participants. (Fig. C)
Clinical Implications
These findings indicate that tirzepatide modulates both metabolic and hedonic drivers of eating behavior. By reducing appetite, caloric intake, and cravings for highly palatable foods, tirzepatide supports meaningful weight reduction beyond lifestyle intervention alone. This dual mechanism—targeting energy regulation and hedonic responses—may help patients achieve and maintain clinically significant weight loss, particularly in individuals struggling with food cravings that impede dietary adherence.
Conclusion
In this phase 1 trial, tirzepatide not only promoted substantial weight loss and reduced energy intake but also significantly decreased food preferences and cravings. These effects highlight the role of tirzepatide in attenuating the appetitive and hedonic drives that contribute to obesity, offering a compelling mechanism for its robust efficacy in weight management.
Reference
Kennedy SF, Knights A, Ravussin E, Sanchez-Delgado G, Nishiyama H, Qian HR, Pratt EJ, Milicevic Z, Haupt A, Coskun T, Martin CK. Impact of tirzepatide treatment on participant-reported food craving and food preference: Secondary analyses of a phase 1 randomised controlled trial in people with obesity with dietary restriction. Diabetes Obes Metab. 2025 Aug 28.
